
Research groups & topics
Nano-translational laboratory – Alexandre Detappe
Molecular Mechanisms and Targeted Therapeutic Intervention for Neuroprotection – Dominique Bagnard
Chemogenomic and Medicinal Chemistry – Frédéric Bihel
Immune-microenvironment interaction in health and disease – Christopher Mueller
Integrative Chemical Biology – Dominique Bonnet
BioFunctional Chemistry – Alain Wagner
Poly (ADP-ribosyl)ation and genome integrity – Françoise Dantzer
Platform of chemical biology – Pascal Villa
Neuroimunology and peptide therapeutics – Sylviane Muller
Immune-microenvironment interaction in health and disease – Christopher Mueller
Integrative Chemical Biology – Dominique Bonnet
Chemogenomic and Medicinal Chemistry – Frédéric Bihel
Platform of chemical biology – Pascal Villa
RCPGs, Pain and inflammation – Frédéric Simonin
Integrative Chemical Biology – Dominique Bonnet
Chemogenomic and Medicinal Chemistry – Frédéric Bihel
Platform of chemical biology – Pascal Villa
Neuroimunology and peptide therapeutics – Sylviane Muller
Molecular Mechanisms and Targeted Therapeutic Intervention for Neuroprotection – Dominique Bagnard
Chemogenomic and Medicinal Chemistry – Frédéric Bihel
Platform of chemical biology – Pascal Villa
Neuroimunology and peptide therapeutics – Sylviane Muller
Chemogenomic and Medicinal Chemistry – Frédéric Bihel
Platform of chemical biology – Pascal Villa
Chemogenomic and Medicinal Chemistry – Frédéric Bihel
Platform of chemical biology – Pascal Villa
Molecular Imaging – Frédéric Boisson
Platform of chemical biology – Pascal Villa
BioOrganic Mass Spectrometry- Sarah Cianférani
Research groups
Nano-translational laboratory
The research laboratory of Prof. Alexandre DETAPPE is located at the Institut de Cancérologie Strasbourg Europe (ICANS) and is affiliated to CNRS UMR7178 and to the ITI Drug Discovery and Development Institute. Its overarching goal is to improve cancer treatment with biotechnology and materials chemistry, employing a materials science-centric approach to develop new therapies to :
- reduce the side effects induced by current chemotherapies
- control the modulation of the immune system,
- assess in vivo the tumor response and the immune system response to novel cancer therapies.
More specifically, we develop novel smart drug delivery systems, immunonanotherapies, supercharged antibody drug conjugates, and novel small molecules. Our expertise focuses on multiple myeloma and breast cancer models, however, we explore novel research opportunies as well as they arise. Our first focus is to translate all of our preclinical work into clinical trials, with the ultimate aim of improving cancer patients treatments. With the expertise of our clinicists, we are currently validating novel nano-based therapeutics for tumor sensitization, novel biosimilars for breast cancer treatments, and novel monoclonal antibodies in various ongoing Phase I/II clinical trials.
UMR 7178 CNRS-Unistra, Institut de Cancérologie Strasbourg Europe (ICANS),
17 Rue Albert Calmette, 67200 Strasbourg
a.detappe (at) icans.eu
Neuroimunology and peptide therapeutics
The research laboratory of Prof. Sylviane Muller is located at the Institut de science et d’ingénierie supramoléculaire (ISIS) in Strasbourg. It depends on the CNRS-Unistra Unit Biotechnology and cell signalling (Ecole supérieure de biotechnologie de Strasbourg). Sylviane Muller co-leads the research team Neuroimunology & peptide therapy, which is focused on the understanding of misdirected immune responses occurring in autoimmune and inflammatory diseases, and on the discovery of new druggable molecules designed to specifically control these disorders. More precisely, the team members :
- decipher the cellular and molecular immune circuits that are altered in patients and murine models with a special focus laid on the lysosomal autophagy processes that are dysregulated in these settings
- study the behavioural disturbances that progressively occur in model mice with neurological autoimmune diseases
- develop strategies that specifically target the identified defective immune processes. The peptide P140 discovered in the team is currently evaluated in phase III clinical trials for Lupus. All these questions are investigated using approaches of molecular and cellular biology, imaging, immunochemistry, peptide chemistry, material chemistry for molecule targeting/vectorization and behavioural approaches in animal.
Biotechnologie et signalisation cellulaire (UMR7242 CNRS-Unistra)
300 boulevard Sébastien Brant, CS 10413, 67412 ILLKIRCH CEDEX
sylviane.muller (at) unistra.fr

Group leader :
Dominique Bagnard
Molecular Mechanisms and Targeted Therapeutic Intervention for Neuroprotection
Therapeutic innovation involves deciphering the molecular interactions that regulate cellular functioning in order to develop drugs capable of modulating these interactions and correcting their dysfunctions. This concept of molecular interactions takes on a particular dimension with the membrane proteins that provide the interface between the extracellular and intracellular environment, which is an essential site for the transmission of signals controlling the cell. Dr. Bagnard’s research laboratory is working on the development of peptide compounds that act as interfering agents modulating the interactions between membrane proteins. These peptides target the transmembrane domains of membrane receptors and have already demonstrated their therapeutic potential for the treatment of cancers such as glioblastoma or metastatic breast cancer but also in the case of demyelinating diseases such as multiple sclerosis. With more than fifteen years of experience in this unique strategy, the laboratory has developed a know-how materialized by a platform for the design and preclinical evaluation of these therapeutic transmembrane peptides applicable to all forms of therapeutic compounds. From the use of in silico modeling to in vivo animal models of pathologies, the laboratory has the expertise and comprehensive technical means to obtain patentable compounds with a high transfer potential. The work carried out also takes into account the need for molecular tools for predicting the evolution of a disease or the response to a treatment. The molecular tools developed in the laboratory aim to validate experimental treatments while contributing to the monitoring of the therapeutic response.
INSERM ERL 1321 – Biopathologie de la Myéline, Neuroprotection et stratégies thérapeutiques (Dir : Pr G. Mensah-Nyagan),
Site d’Illkirch Pôle API Bâtiment E – 300 Boulevard S. Brant
bagnard (at) unistra.fr

Group leader :
Sarah Cianférani
BioOrganic Mass Spectrometry
The BioOrganic Mass Spectrometry laboratory (LSMBO) belongs Analytical Sciences Department of the Institut Pluridisciplinaire Hubert Curien (IPHC, www.iphc.cnrs.fr, CNRS Université de Strasbourg UMR7178). The LSMBO is hosting an IBiSA-certified proteomic platform (Strasbourg Grand Est Proteomic Platform) and is the Strasbourg node of the French Proteomic Infrastructure (ProFI, www.profiproteomics.fr). The LSMBO is a 35 scientists team that has a 30 year-experience in the structural study of peptides and proteins by mass spectrometry. The laboratory is renowned for developing new methods in quantitative proteomics, bioinformatics for proteomics and structural mass spectrometry for the analysis of protein/protein interactions in large complexes. Besides that, long term collaborations with several pharmaceutical companies cover topics such as full characterization of natural and/or recombinant proteins used in human therapy, toxicoproteomics, protein/drug interaction.
Field of expertise:
- Sample preparation for proteomic analysis (1D or 2D gels, liquid digestion, PTMs enrichment…)
- Quantitative label-free proteomics
- Targeted quantitative proteomics (LC-SRM/PRM)
- PTM identification and quantification (phosphorylation, etc.)
- Bioinformatics for proteomics (de novo sequencing),
- Interactomics
- Recombinant protein analysis (eg monoclonal antibodies)
- Native MS and Ion Mobility of noncovalent complexes
- HDX-MS
- cross-linking MS
UMR 7178 CNRS-Unistra, Institut Pluridisciplinaire Hubert Curien (IPHC),
23 rue du Loess, BP 28, 67037 Strasbourg Cedex
sarah.cianferani (at) unistra.fr

Group leader :
Frédéric Simonin
RCPGs, Pain and inflammation
G protein coupled receptors (GPCRs) represent the largest family of membrane receptors in eukaryotes with more than 800 members in humans. They are stimulated by a wide variety of extracellular signals and are involved in many physiological and pathophysiological processes including neurotransmission, cell division and differentiation, chemotaxis, inflammation and pain. As such, they represent preferred targets for the development of new drugs. Our team is particularly interested in the role of different GPCRs in the development of pain and associated inflammatory processes as well as the mechanisms that regulate GPCR signaling. Our approaches include the development of pharmacological (antagonists) and genetic (KO mouse) tools for these different targets as well as new therapeutic strategies for the treatment of pain.
UMR 7242 CNRS-Unistra, Ecole Supérieure de Biotechnologie de Strasbourg (ESBS),
300 Boulevard Sébastien Brant, 67412 Illkirch
frederic.simonin (at) unistra.fr

Group leader :
Frédéric Bihel & Esther KELLENBERGER
Chemogenomic and Medicinal Chemistry
Using artificial intelligence as well as cutting-edge concepts in organic and medicinal chemistry, the CCM team designs, synthesizes and functionalizes innovative molecular scaffold applied to drug discovery.
UMR 7200 CNRS-Unistra, Faculté de Pharmacie,
74 route du Rhin, 67401 Illkirch, cedex, FRANCE
frederic.bihel (at) unistra.fr

Group leader :
Frédéric Boisson
Molecular Imaging and Radiobiology
The team offers all the expertise needed for instrumental development, quantification and signal processing in the field of imaging, and more specifically the “nuclear” imaging modalities such as PET and SPECT. This expertise is also strengthened by other internal skills in radiochemistry through the labeling of molecules (antibodies, peptides, macro- and small molecules) with various isotopes dedicated to PET and SPECT imaging produced thanks to the Cyrcé cyclotron.
UMR 7178 CNRS-Unistra ,Institut Pluridisciplinaire Hubert Curien (IPHC),
23 rue du Loess, BP 28, 67037 Strasbourg Cedex
frederic.boisson (at) iphc.cnrs.fr

Group leader :
Christopher Mueller
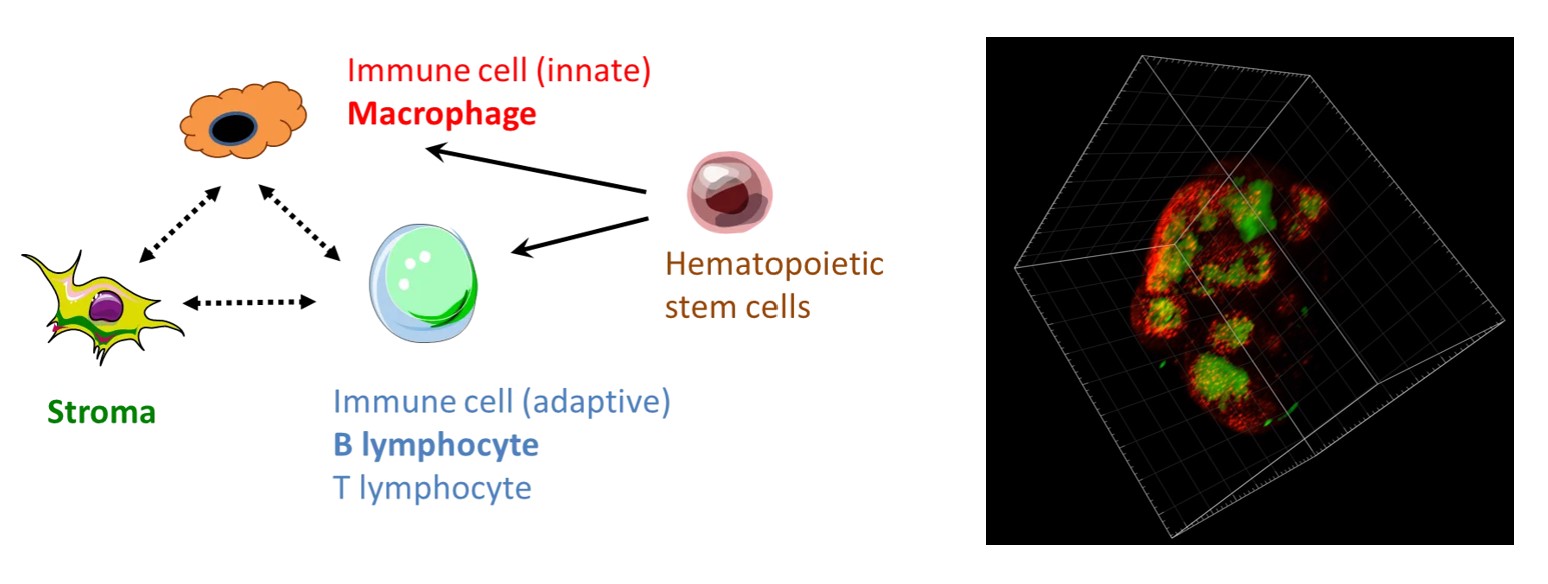
Immune-microenvironment interaction in health and disease
For example, we have recently shown that lymphatic endothelial cells create a niche for a subtype of lymph node macrophages involved in the anti-infectious and anti-cancer immunity.
In order to model diseases in humans and towards personalized medicine, the laboratory is reconstructing organoids in vitro, such as immuno-competent and innervated human skin. In addition, we have exploited the natural affinity of arboviruses for human skin immune cells to generate vectors targeting macrophages and dendritic cells.
- Isolation et cultures en 2D et 3D de cellules murines et humaines primaires
et de cellules humaines neuronales dérivées des iPSCs. / Isolation and 2D + - Transparisation et microscopie à fluorescence d’organe entier
c.mueller (at) ibmc-cnrs.unistra.fr

Group leader :
Pascal Villa
Platform of chemical biology
PCBIS offers its services to public and private laboratories
- Assay development : The services “High Throughput Screening” and “Target Libraries” set up miniaturized and automated assays , produce and validate biological models (target based or cell based). This can be done from cloning to protein expression (soluble proteins and stable or transient cell lines)
- High Throughput Screening (HTS) : The service “HTS” validates and runs automated screening of chemical libraries in order to identify active compounds.
- Microfluidics : The service “Microfluidics” develops new models and tools in order to perform innovative lab on a chip experiments.
- Chemical libraries : The service “Chemical librairies” manages compound collections, performs structure activity relationship (SAR) analysis and contributes to hit optimization.
- ADME-Tox : The service “TechmedILL” establishes the physicochemical and pharmacokinetics of compounds (solubility, log D, pKa, chemical and plasmatic stability, membrane permeability, metabolism, pK in vivo…) and cellular toxicity.
- Training : our engineers train and supervise students and researchers who want to use our technologies and facilities..
- Identification de candidats médicaments par des techniques innovantes de fluorescence en tests cellulaires et moléculaires miniaturisés
- Analyse des propriétés pharmacologiques et précliniques des composés
- Modèles animaux (asthme, inflammation…)
pvilla (at) unistra.fr

Group leader : Françoise Dantzer
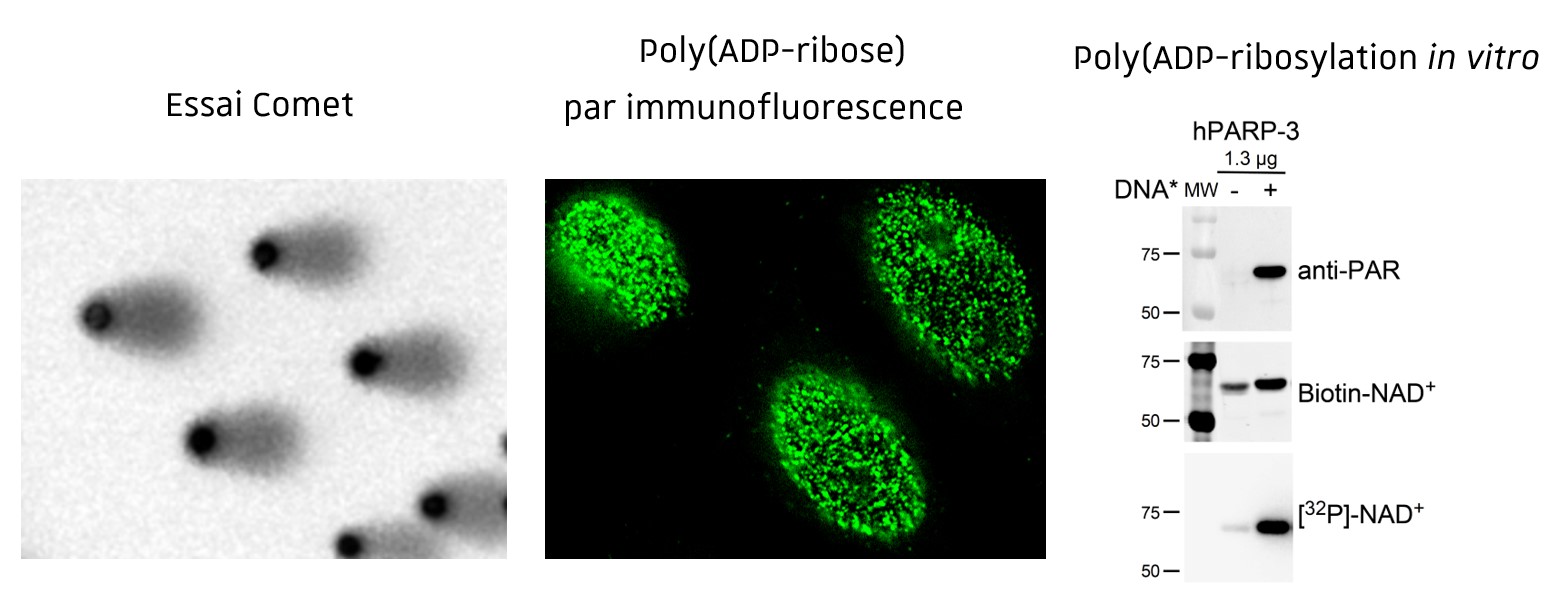
Poly (ADP-ribosyl)ation and genome integrity
The research of our team aims to clarify the complex role of poly(ADP-ribosyl)ation in the cellular processes controlling genome integrity and tumorigenesis. Previous work of the team has focused on dissecting the properties of the enzymes catalyzing the synthesis of poly(ADP-ribose) (PAR) in response to DNA damage, the poly(ADP-ribose) polymerases PARP1 and PARP2, and the enzyme degrading PAR, known as poly(ADP-ribose) glycohydrolase (PARG).
We defined PARP1 as a sensor of DNA strand breaks, playing essential functions in the spatial and temporal organization of their repair through the local synthesis of PAR and recruitment of DNA repair enzymes at the site of the lesion. Its inhibition is currently used in clinical trials to potentialize the cytotoxic action of antitumoral drugs and radiation therapy or to target repair deficient cancers (BRCAmut). PARP2 has also been shown to contribute to genome integrity. By combining biochemical studies and characterization of PARP1 and PARP2-deficient cellular and animal models, we uncovered redundant functions of both proteins in DNA repair and more specific activities in the regulation of transcription, chromatin organization and cell differentiation. Finally, we identified prime functions of PARG to prevent detrimental accumulation of PAR upon irradiation and prolonged replication stress.
These last years, we undertook the biochemical and functional characterization of novel PARP members, PARP3 and PARP9. While PARP9 is described as a novel actor in stress-induced double-strand break repair, our PARP9 knockout mouse model does not show obvious defects in the repair of programmed double-strand breaks. We found that PARP3 plays important functions in cell response to double-strand breaks, mitotic progression, epithelial-mesenchymal transition and stemness in breast cancer.
Our challenge today is to further dissect the biochemical and biological properties of PARP3 in double-strand break repair and tumorigenesis in breast cancer, glioblastoma and pancreas cancer. We also explore its contribution in cell differentiation events during neurogenesis and muscle regeneration. We develop these projects combining biochemistry, cellular approaches and molecular biology with the generation and functional characterization of loss-of-function cellular models (with a particular interest for stem cells) and animal models (Cripsr/Cas 9, knockout mouse models).
Techniques d’étude de l’intégrité du génome et des mécanismes de réparation in vitro (Essais de Poly(ADP-ribosyl)ation), in cellulo (SCE, Essais Comet, Essais Tunel, cinétique des foyers gH2AX, 53BP1, BRCA1, RAD51, poly(ADP-ribose) par immunofluorescence, modèles cellulaires d’évaluation de la réparation) et in vivo (gH2AX, 53BP1, BRCA1, RAD51, poly(ADP-ribose) par immunohistochimie sur coupes de tissus).
francoise.dantzer (at) unistra.fr

Group leader : Dominique Bonnet
Integrative Chemical Biology
Our team belongs to the Laboratory of Therapeutic Innovation (UMR7200, Faculty of Pharmacy of Illkirch) and is member of the Strasbourg Drug Discovery and Development Institute (IMS), the Pole of Excellence ‘Frontier Research in Chemistry’ and the Graduate School of Pain (EURIDOL).
- Patented “FluoroPEP” technology1.
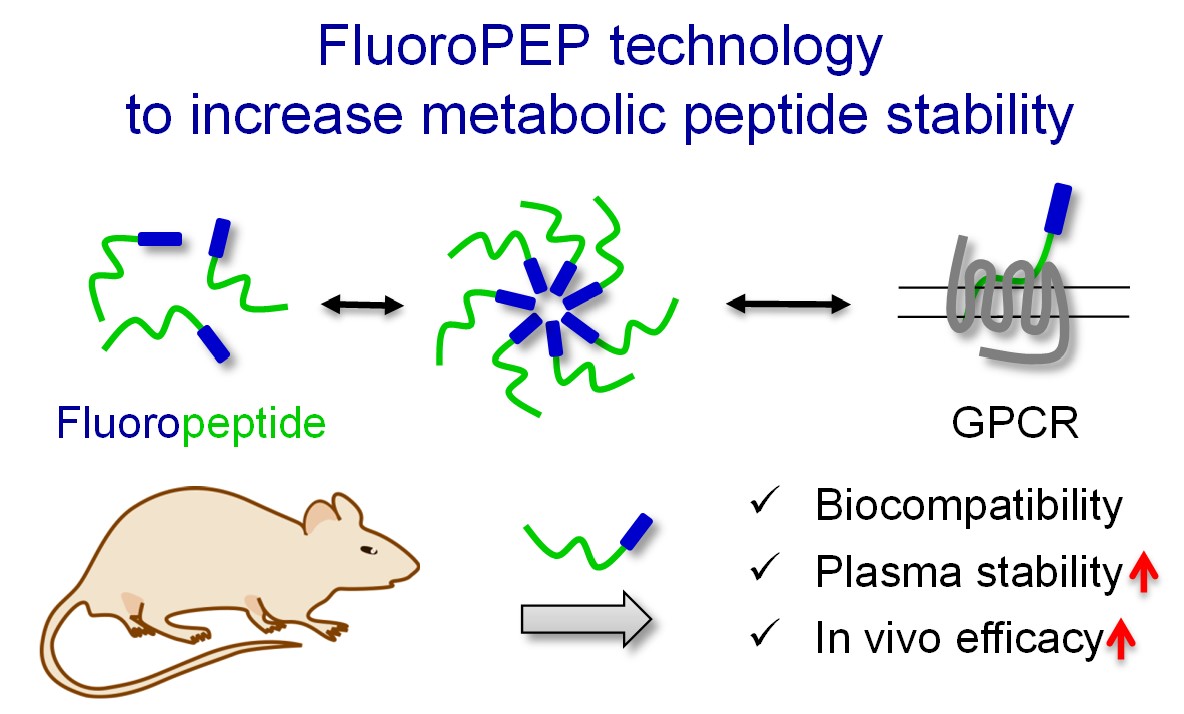 Figure. Innovative approach to increase plasmatic stability and in vivo efficacy of peptides for therapeutic applications.
References:
[1] (a) Esteoulle L.; Simonin F.; Bonnet D. Metabolically stable spexin peptide analogs. WO2017216360; 21-12-2017; (b) Iturrioz X.; Llorens-Cortes C.; Bonnet D. Metabolically stable peptide analogs. WO2017216359; 21-12-2017; (c) Iturrioz, X.; Llorens-Cortes, C.; Bonnet D. Metabolically stable apelin analogs in the treatment of disease mediated by the apelin receptor. WO2016/102648, 30-06-2016.
[2] (a) Flahault, A. et al. NatCommun., 2021, 12, 1-14; (b) Jourdain de Muizo, C. et al. ChemBioChem, 2020, 1-6; (c) Gerbier, R. et al. FASEB J., 2017, 31, 687-700.
Figure. Innovative approach to increase plasmatic stability and in vivo efficacy of peptides for therapeutic applications.
References:
[1] (a) Esteoulle L.; Simonin F.; Bonnet D. Metabolically stable spexin peptide analogs. WO2017216360; 21-12-2017; (b) Iturrioz X.; Llorens-Cortes C.; Bonnet D. Metabolically stable peptide analogs. WO2017216359; 21-12-2017; (c) Iturrioz, X.; Llorens-Cortes, C.; Bonnet D. Metabolically stable apelin analogs in the treatment of disease mediated by the apelin receptor. WO2016/102648, 30-06-2016.
[2] (a) Flahault, A. et al. NatCommun., 2021, 12, 1-14; (b) Jourdain de Muizo, C. et al. ChemBioChem, 2020, 1-6; (c) Gerbier, R. et al. FASEB J., 2017, 31, 687-700.
- Patented “Turn-ON” technology1.
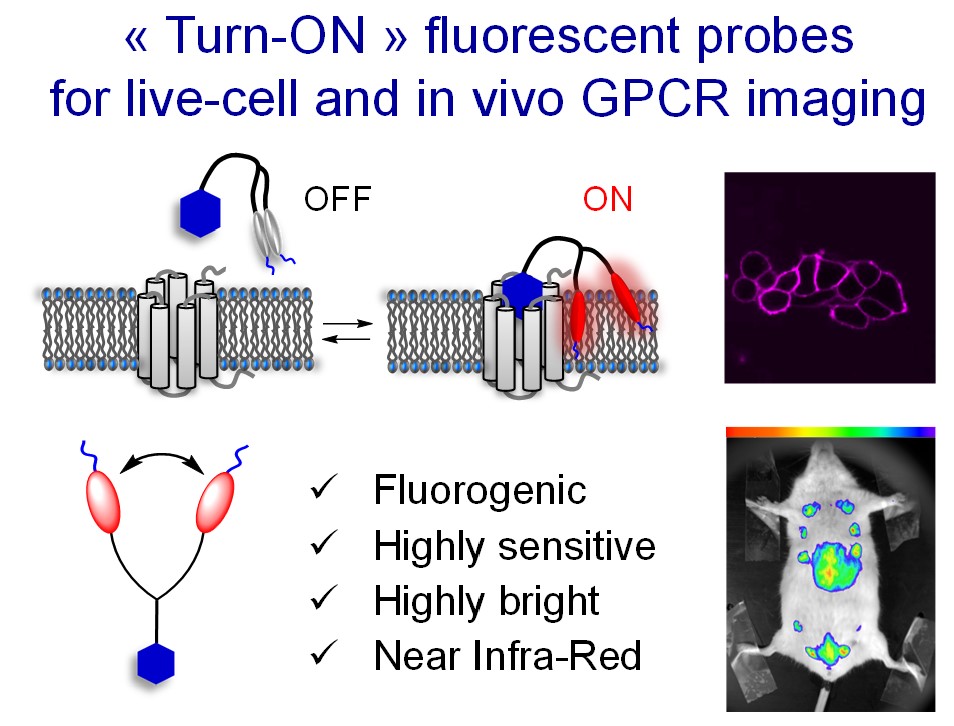 Figure. New concept of fluorogenic dimer ligands for the background-free fluorescence imaging of GPCR.
References:
[1] Karpenko, I.; Collot, M.; Klymchenko, A.; Bonnet, D. EP20305479.6, 12-05-2020
[2] Karpenko, I.A. et al. J. Am. Chem. Soc., 2015, 137, 405-412.
[3] Esteoulle, L. et al. Chem. Sci. 2020, 11, 6824-6829.
Figure. New concept of fluorogenic dimer ligands for the background-free fluorescence imaging of GPCR.
References:
[1] Karpenko, I.; Collot, M.; Klymchenko, A.; Bonnet, D. EP20305479.6, 12-05-2020
[2] Karpenko, I.A. et al. J. Am. Chem. Soc., 2015, 137, 405-412.
[3] Esteoulle, L. et al. Chem. Sci. 2020, 11, 6824-6829.dominique.bonnet (at) unistra.fr

Group leader : Guilhem Chaubet
BioFunctional Chemistry
We are primarily developing our research along three directions:
Bio-specific chemical reactions: for precise stoichiometric conjugation and controlled delivery of drugs or payloads. Control of conjugation stoichiometry opens prospects toward novel format of hybrid bimolecular constructs for drug delivery or biophysical studies.
In vivo chemistry: to uncover the rules that guide chemical reactions in complex biofluids taking into account reagent transport, bio-distribution and pharmacokinetics. It opens new opportunities toward alternative therapeutic and chemo-omics approaches via in vivo modification of biotics (metabolites) or xenobiotics (drugs, imaging agents).
Single Cell Omic: by developing novel biomolecule capture system and phase inversion microfluidic technologies we paved the way toward advanced single cell multi-omic approaches (transcriptomic, proteomic, secretomic, surfacomic). This technology provides a unique insight into cancer heterogeneity and differentiation processes.
UMR 7199 CNRS-Unistra, Laboratoire de Conception et Application de Molécules Bioactives
Faculté de pharmacie, 74 route du Rhin, 67401 Illkirch Cedex

